The Naralie M. LaPrade Maryland Medical Cannabis Commission named, yesterday, the 15 Growers and 15 Processors who have been awarded Stage One license pre-approvals. The Commission’s actions mark a giant step forward for the medical cannabis program in Maryland.
Dr. Paul W. Davies, Chairman of the Maryland Medical Cannabis stated, “I’m excited that we have a great number of outstanding companies willing to help sick people in Maryland.” Those who will receive the cannabis license consists of a diverse group of principals, executives and corporate officers. This list also includes Maryland residents associated with long-time established Maryland businesses, entrepreneurs, women and minority-operated enterprises. Seven of the Processors are affiliated with Growers who received Stage One pre-approvals.
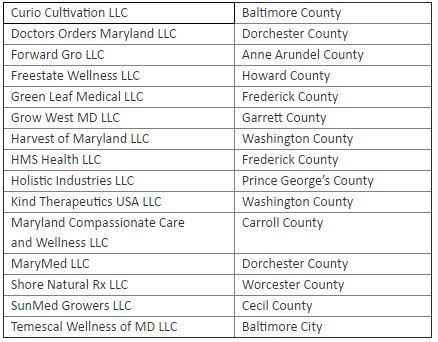
Grower entities who will receive a cannabis license:
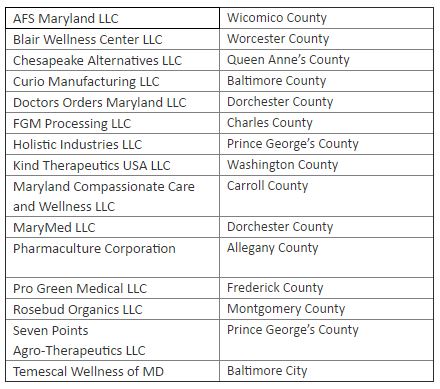
Processors receiving a cannabis license:
The Maryland Medical Cannabis Commission’s Executive Director, Patrick Jameson stated, “Now that the Commissioners have made their selections, the real work begins for these companies. We will implement a rigorous Stage Two background and financial due diligence process for these entities prior to issuing a license. A pre-aproval is not a license. I truly look forward to facilitating this nascent state wide industry and working with local, city, and county jurisdictions and with these the principals of these organizations.” The Commissioners will vote for licensure once compliance with the regulatory requirements is complete.
The locations of the Growers and Processors span 16 counties and Baltimore City, including Anne Arundel, Baltimore, Carroll, Dochester, Frederick, Garrett, Howard, and more. Many grower applicants who were highly qualified were not pre-approved due to statutory limitations on the number of growers.
The Growers will cultivate a variety of strains ranging in cannabinoid content from a low to high spectrum of Tetrahydrocannabinol (THC) and Cannabidiol (CBD), and the Processors will manufacture a wide variety of cannabis-infused products containing high and low CBD and THC extracts and i n a variety of modes of administration. These pharmaceutical grade products will include oral forms such as oils, pills, capsules, tinctures, sublingual sprays; inhaled products and topical forms such as ointments, salves, and transdermal patches.
MMCC also commissioned the Towson University, Regional Economic Studies Institute “RESI” to coordinate the review of the Dispensary Applications by subject matter experts and to utilize the same process as for the Grower and Processor applications such as compiling the scores and application rankings. The Commissioners will review and vote on the Dispensaries with medicine tentatively becoming available by the summer of 2017.
Dr. Davies continues, “While we are aware there is a public interest in the rankings of the Grower and Processor applications, the rankings were merely used as an organizational tool. The Commission will list the entities in alphabetical order. All of the chosen business entities have an equal opportunity to complete the licensing process. At this stage, I am very happy with the Commissioners’ choices.”
Maryland received 145 grower applications, 124 processor applications and 811 dispensary license applications. Again, MMC commissioned RESI to contract with third-party subject matter experts from around the United States to evaluate the applications using a double-blinded process. RESI assigned unique identifying information such as individual or entity applicant names and locations. Once evaluated and scored by the subject matter experts, RESI compiled the scored and ranked the applications. The score and ranked redacted Grower and Processor applications were then provided to Commissioners for review and final selection.
The 15 Grower and Processor potential licensees will continue on to Stage Twi if the approval process, which will include extensive financial due diligence and background investigations by the MMC of all those named in the original application and any other principals. Each entity that was issued a pre-approval will have 365 days from date of notification to complete all necessary steps to obtain a formal license and to request final inspection by the Commission. These steps include completing regulatory requirements, raising capital, acquiring real estate, securing local zoning approvals, construction of facilities, installation of equipment, and the hiring and training of staff.
All applicants, including those who did not receive pre-approvals, were notified as to the status of their application by email and U.S. mail on Monday.



